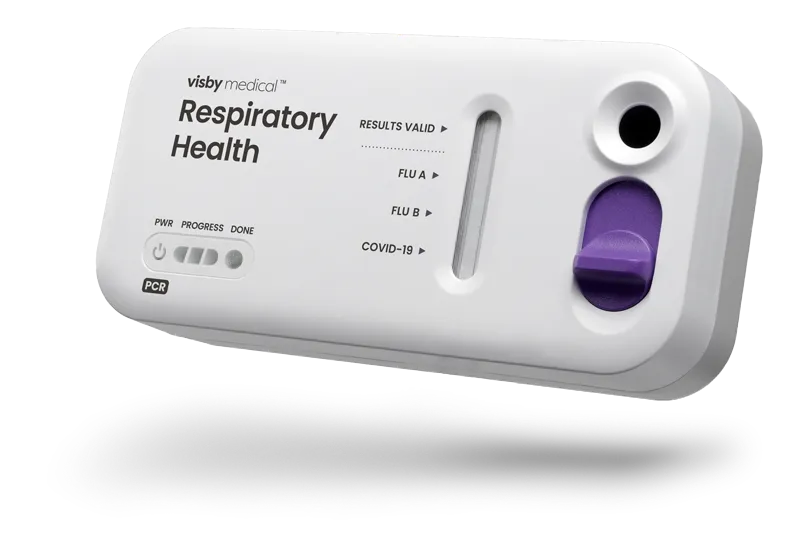
Accurate PCR results without the instrument
Test to treat in a single
patient visit.
Instrument-free PCR for respiratory health

Easy to use
One swab, three targets: COVID-19, influenza A and influenza B

Affordable
No maintenance or service contracts

Scalable
Easy to run multiple test devices at the same time
Three critical targets
COVID-19 and flu are highly contagious respiratory illnesses. The Visby Medical Respiratory Health Test can differentiate between the causative viruses and give answers to help guide providers toward the most effective treatments.

Flu A
According to the CDC, molecular assays, including RT-PCR, are recommended for testing respiratory tract specimens from hospitalized patients because of their high sensitivity and high specificity.
Flu B
The Infectious Diseases Society of America (IDSA) recommends use of rapid influenza molecular assays over rapid influenza diagnostic tests (RIDTs) for detection of influenza viruses in respiratory specimens of outpatients.
COVID-19
The current gold standard is to perform reverse-transcription polymerase chain reaction (PCR) on nasopharyngeal samples. Best-in-class assays demonstrate a limit of detection (LoD) of approximately 100 copies of viral RNA per milliliter of transport media.
Scale
as surges happen
The instrument free Visby Medical Respiratory Health Test allows testing for COVID-19 and flu where it’s most effective, at the point of care.

How to use the Visby Medical Respiratory Health Test
Less than 15 seconds of hands-on time. Results in under 30 minutes.
Product details
 True PCR results that detect and differentiate RNA from the viruses that cause flu and COVID-19, in under 30 minutes with lab accuracy in the palm of your hands.
True PCR results that detect and differentiate RNA from the viruses that cause flu and COVID-19, in under 30 minutes with lab accuracy in the palm of your hands.


| Limit of Detection (LOD) | Influenza A | Influenza B | SARS-CoV-2 |
| Nasopharyngeal Swab | Influenza A/HINI 2009, Brisbane/02/18 106 copies/ swab Influenza A/H3N2, Kansas/14/2017 125 copies/ swab | Influenza B/Washington/02/19 728 copies / swab Influenza B/Oklahoma/10/2018 778 copies / swab | SARS-CoV-2 (USA-WA1/2020) 100 copies swab |
| Positive Percent Agreement (PPA) | 97.9% | 96.9%* | 97.4% |
| Negative Percent Agreement (NPA) | 99.3% | 100%* | 98.8% |
* Data is a combination of prospective fresh specimens (NP and AN), banked specimens (NP), and surrogate specimens (NP).
Downloads
510(k) Respiratory Health Test
EUA Respiratory Health Test
Additional Information
Downloads
Visby Medical developed PCR easy enough to be used by almost anyone, in any CLIA-waived setting. Below are resources to help run a successful test.

This product has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection and differentiation of nucleic acid from SARS-CoV-2, influenza A, and influenza B, not for any other viruses or pathogens; and the emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.