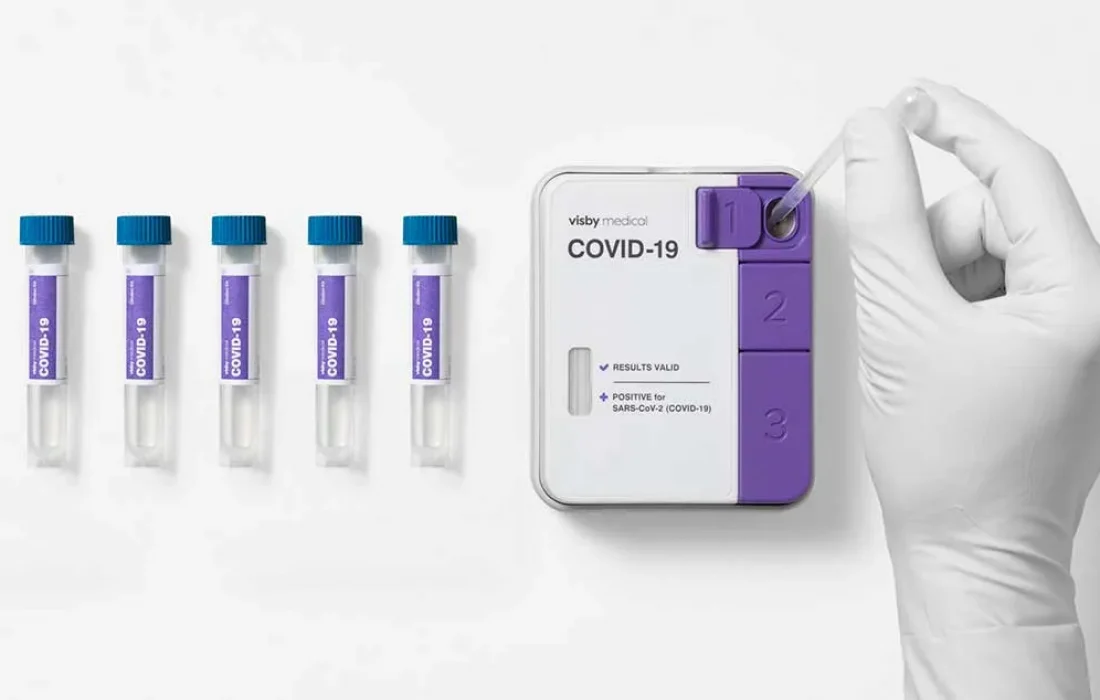
First instrument-free PCR test to detect the SARS-CoV-2 virus can now be used to pool up to five patient samples using a single test
San Jose, CA – September 13, 2021 – Visby Medical™ announced today that its instrument-free Reverse Transcription (RT)-Polymerase Chain Reaction (PCR) COVID-19 test was granted Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) for testing pooled patient samples in high-complexity Clinical Laboratory Improvement Amendments (CLIA)-certified laboratories. This authorization extends the previous EUA for single patient sample testing at the point of care. Furthermore, this validates the flexibility and adaptability of the Visby Medical innovative, palm-sized PCR technology by expanding its proven capabilities from individual, single-use testing to pooled testing of up to 5 patient samples at once.
According to the U.S. Centers for Disease Control and Prevention (CDC), more than 32 million COVID-19 tests have been performed in the U.S. alone over the past month.1 Pooling patient samples with the Visby test can help increase overall lab testing capacity without additional tools or resources, while maintaining both accuracy and speed by returning results in less than 30 minutes.
“With the SARS-CoV-2 virus ravaging the country, Visby remains steadfast in meeting the urgent needs of the community, its partners, and the changing market dynamics with our instrument-free PCR COVID-19 test,“ said Teresa Abraham, PhD, Director, Medical Affairs at Visby Medical. “Thanks to this EUA, laboratories can meet increasing testing demand by analyzing up to five patient samples at once, allowing for significantly increased efficiency and significantly decreased testing costs, particularly in low prevalence settings.”
How Sample Pooling Works
The Company’s COVID-19 test pooling protocol enables high-complexity clinical laboratories to combine up to five patient samples into a single device for processing. A negative result means that all five individuals have tested negative for SARS-CoV-2. A positive result triggers all five samples to be re-tested individually to determine which patient or patients are infected.2
By shrinking PCR technology to palm-sized dimensions, the Visby Medical platform provides fast, accurate results. This single-use, instrument-free device quickly identifies COVID-19 infections, which is especially useful in communities with limited access to testing.

About Visby Medical™
Visby Medical is a diagnostics company that is transforming the order of diagnosis and treatment for infectious diseases. The Company’s proprietary technology development program culminated in the world’s first instrument-free, single-use PCR platform that fits in the palm of your hand and rapidly tests for serious infections. Originally developed for sexually transmitted infections, the Company’s FDA-cleared, CLIA-waived Sexual Health Click Test for women returns accurate results within 28 minutes. The Visby Medical technology is also helping to fight the global pandemic via the Visby Medical COVID-19 Test, and its robust pipeline includes tests for other infectious diseases. Visby Medical is accelerating the delivery of fast and accurate, palm-sized PCR diagnostics to the point of care, and eventually for use at home.
For more information, visit www.visbymedical.com. Follow Visby Medical on LinkedIn; Facebook, Instagram, and Twitter.
Contact Visby’s Media team for any inquiries: press@visbymedical.com
References:
- Centers for Disease Control and Prevention. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#cases_tests30day. Access August 5, 2021.
- Centers for Disease Control and Prevention. Interim Guidance for Use of Pooling Procedures in SARS-CoV-2 Diagnostic and Screening Testing. https://www.cdc.gov/coronavirus/2019-ncov/lab/pooling-procedures.html. Accessed August 5, 2021.