FDA Grants Emergency Use Authorization for Visby Medical’s COVID-19 RT-PCR Test for Pooled Samples
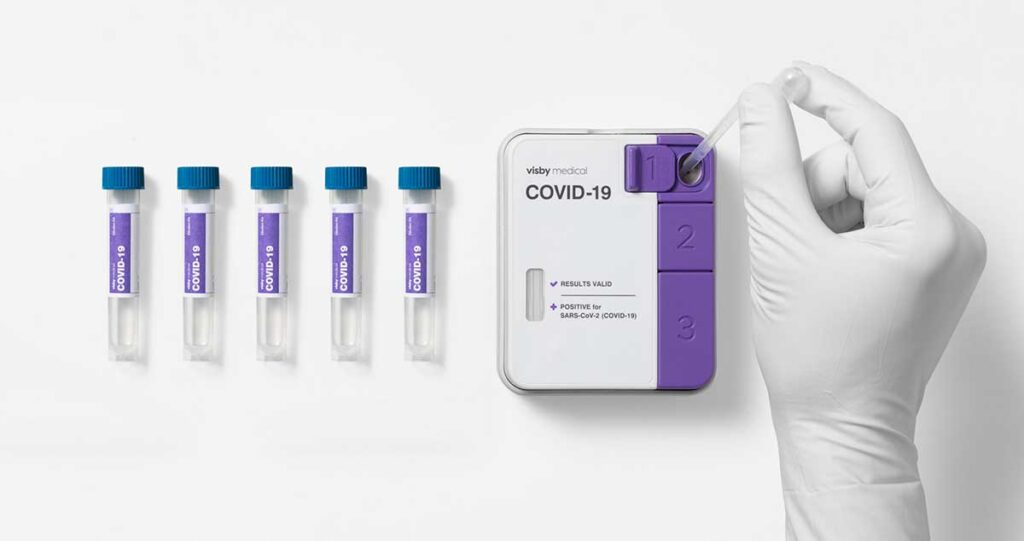
The Visby Medical™ instrument-free Reverse Transcription (RT)-Polymerase Chain Reaction (PCR) COVID-19 test was granted Emergency Use Authorization (EUA) by the FDA
COVID-19 Testing: Its Role in Safely Reopening

The global COVID-19 pandemic touches and disrupts every aspect of life and livelihood across geographies. Despite progress with public health practices and vaccinations, our personal and professional futures face uncertainty. Inconsistent and ambiguous re-opening procedures hinder a robust return to pre-pandemic routines that can restore productivity and economic health.