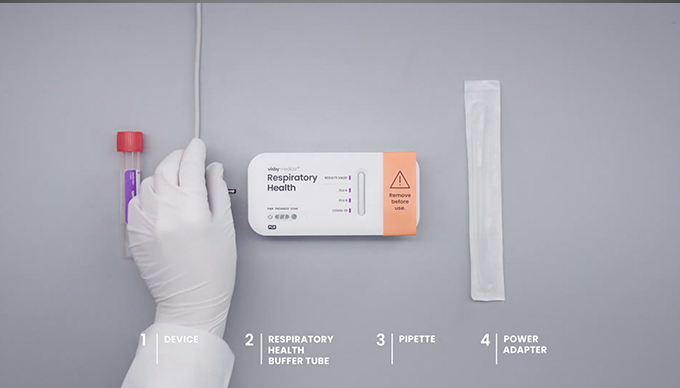
Getting Started
Please view our step-by-step tutorial videos.
Optional training certificate available upon quiz completion.
Quickstart Videos
Playlist
Chapter 1 Tips:
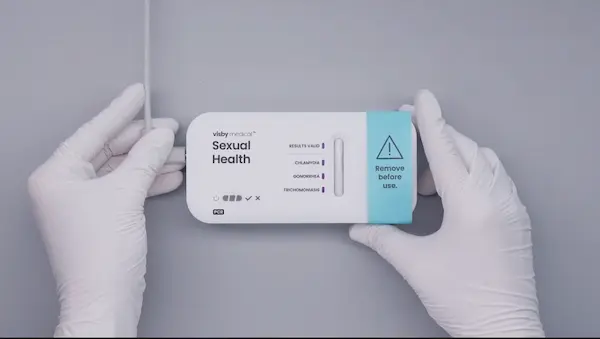
- Do not remove the device from the foil packaging until you are ready to run the test.
- Once a test is plugged in and processing, do not unplug or move the device.
If you’d like to complete a control run during this video training, please have all the supplies nearby:
- 2 Visby Testing Devices (to run controls simultaneously)
- 2 Visby Power Adapters (to run controls simultaneously)
- 2 ZeptoMetrix control vials. (1 positive & 1 negative vial pulled from the refrigerator as close to training time as possible)
- Gloves (best practices)
- Chuck or Paper Towel (to avoid contamination)
Chapter 2 Tips:
Patient Samples:
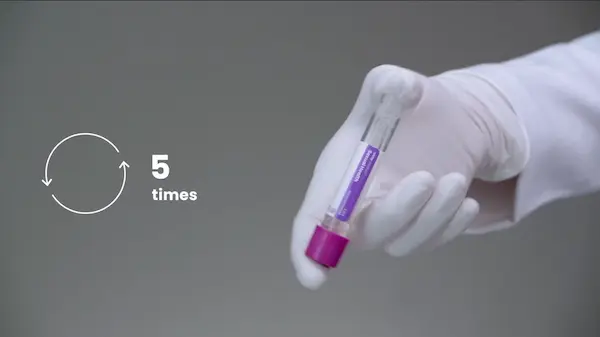
- The patient sample in the Visby Medical Collection Media is stable for 4 hours at room temperature. Mix the sample by gently inverting the specimen 5 times, prior to loading the sample into the device.
- If needed, you may freeze a patient sample for up to 90 days. If using a frozen specimen, allow the sample to thaw for approximately 1-2 hours prior to use. Invert the sample 5 times prior to loading the sample into the device.
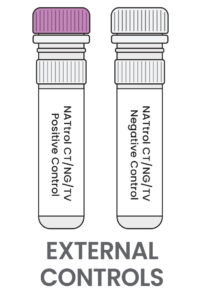 ZeptoMetrix™ Controls:
ZeptoMetrix™ Controls:
- If using a control vial, there is no dilution step and no wait time from the refrigerator. Use a refrigerated control vial as if it were a refrigerated patient specimen. Invert the sample 5 times prior to loading the sample into the device.
- Clinical Lab Managers often have their staff notate on the exterior of the Visby Device Box the date controls were run so that it’s easy to identify boxes ready for use, or new orders that require a control to be run.
Chapter 3 Tips:
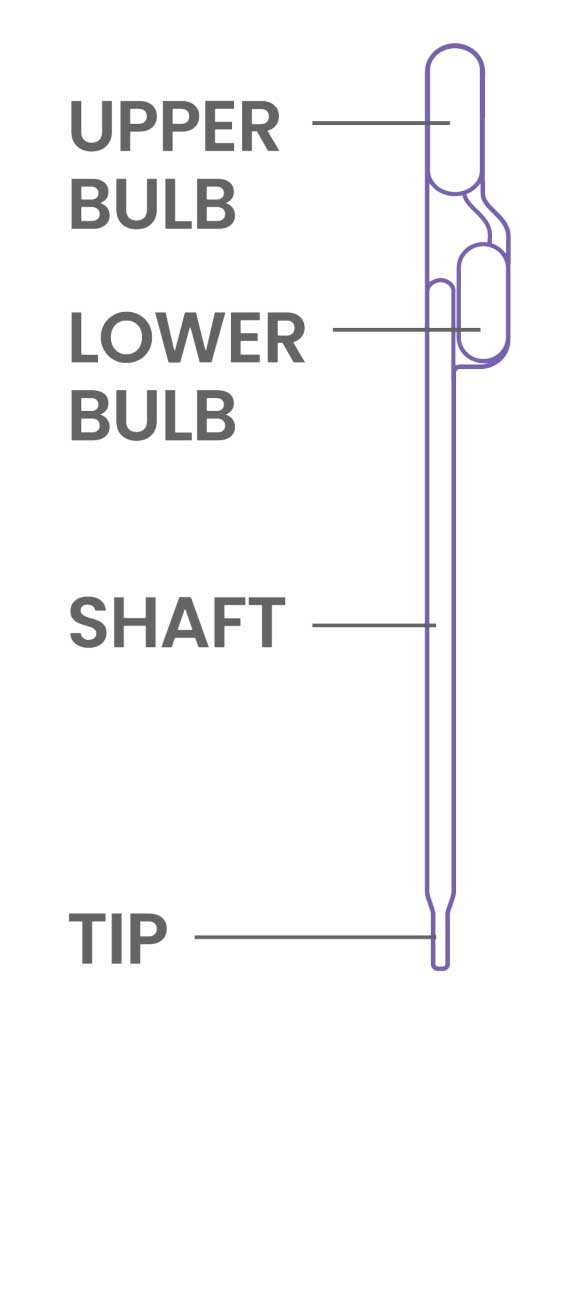
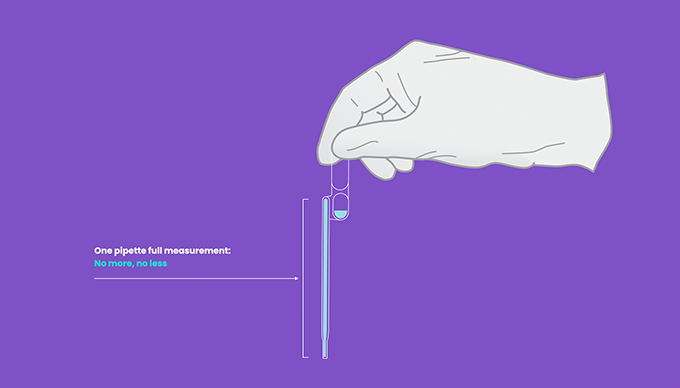
- Treat the pipette similar to an eye dropper, squeeze the upper bulb of the provided pipette prior to submerging the tip to the bottom of the sample tube.
- Do not invert the pipette once the sample is loaded. Doing so may create air bubbles in the shaft.
- Some fluid will remain in the lower bulb of the pipette.
- Once the sample is loaded into the device, keep the upper bulb squeezed as the pipette tip is removed from loading the sample as to not asperate liquid back into the pipette shaft.
- To avoid contamination, do NOT set the pipette down on the counter space. Immediately discard the pipette according to your institution’s guidelines.
Chapter 4 Tips:
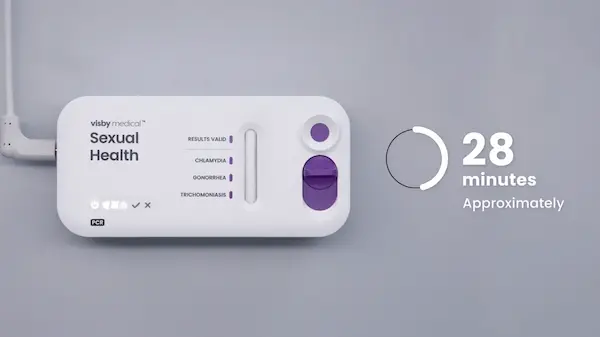
- Once a test is plugged in and processing, do not unplug or move the device.
- The results will be ready in approximately 28 minutes.
If you are watching this video while running a Visby test, please pause now. After 28 minutes, the test you are running should be complete. When you resume watching, begin with Chapter 5.
Chapter 5 Tips:
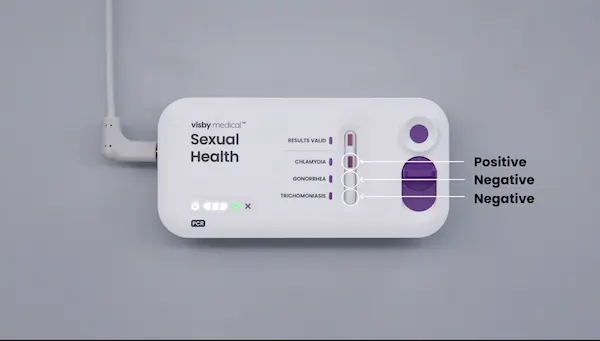
- If a retest is required, use the same patient sample as long as it has been properly stored (≤ 4 hours at room – refrigerated) with a new Visby Medical Sexual Health device and new Visby pipette. Be sure to invert the specimen 5 times prior to loading the sample into the device.
- Dispose of the device and other materials according to your institution’s guidelines.
For more information, please see the Visby Medical Sexual Health Test Instructions for Use. Contact us directly by email support@visby.com or call 1-833-468-4729 (1-833-GoVisby) for any additional questions or support.
Please review all Sexual Health Test chapter videos before taking the quiz.
Quickstart Materials
- Sexual Health – Quick Reference Guide
- Sexual Health – Instructions for Use
- Sexual Health – Collection Instructions for Use
- Sexual Health – Vaginal Self-Collection Instructions – English
- Sexual Health – Vaginal Self-Collection Instructions – Spanish
(more languages available)
- Sexual Health – Vaginal Self-Collection Instructions — (Simplified) Chinese
- Sexual Health – Vaginal Self-Collection Instructions — (Traditional) Mandarin
- Sexual Health – Vaginal Self-Collection Instructions — Japanese
- Sexual Health – Vaginal Self-Collection Instructions — Russian
- Sexual Health – Vaginal Self-Collection Instructions — Marshallese
- Sexual Health – Vaginal Self-Collection Instructions — Portuguese
- Sexual Health – Vaginal Self-Collection Instructions — Haitian-Creole
- Sexual Health – Vaginal Self-Collection Instructions — Arabic
- Sexual Health – Vaginal Self-Collection Instructions — Farsi
- Sexual Health – Vaginal Self-Collection Instructions — Korean
- Sexual Health – Vaginal Self-Collection Instructions — Macedonian
- Sexual Health – Vaginal Self-Collection Instructions — Serbian (Latin)
For all instructional and training materials, please visit our
Playlist
Chapter 1 Tips:
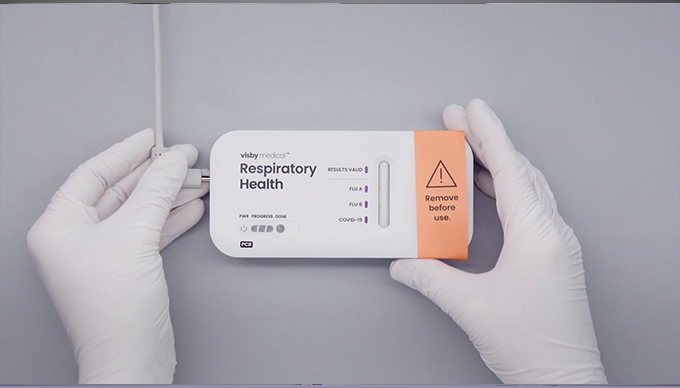
- Do not remove the device from the foil packaging until you are ready to run the test.
- Once a test is plugged in and processing, do not unplug or move the device.
If you’d like to complete a control run during this video training, please have all the supplies nearby:
- Visby Testing Devices (1 x Positive Control, 1 x Negative Control)
- 2 Visby buffer tubes (red cap tubes provided in the same box as the devices)
- 2 Visby Power Adapters (to run controls simultaneously)
- 2 Swabs (1 x Positive Control, 1 x Negative Control. Do not remove packaging until ready to use during training.)
- Gloves (best practices)
- Chuck or Paper Towel (to avoid contamination)
Chapter 2 Tips:
- Do not remove the device from the foil packaging until you are ready to run the test.
- If the power light does not turn on or if the power light blinks, refer to the instructions for use for additional support.
Chapter 3 Tips:
- A patient sample or a control sample is stable in the Visby Respiratory Health Buffer for 2 hours at room temperature or 48 hours at refrigerated temperature.
- For the control swab, gently tap the swab on the bottom of the tube 5 times, then discard the swab in accordance with your institution’s instructions. Screw the cap back onto the tube
- Invert a sample in the Visby buffer tube 5 times prior to loading into the device.
- Clinical Lab Managers often have their staff notate on the exterior of the Visby Device Box the date controls were run so that it’s easy to identify boxes ready for use, or new orders that require a control to be run.
Chapter 4 Tips:
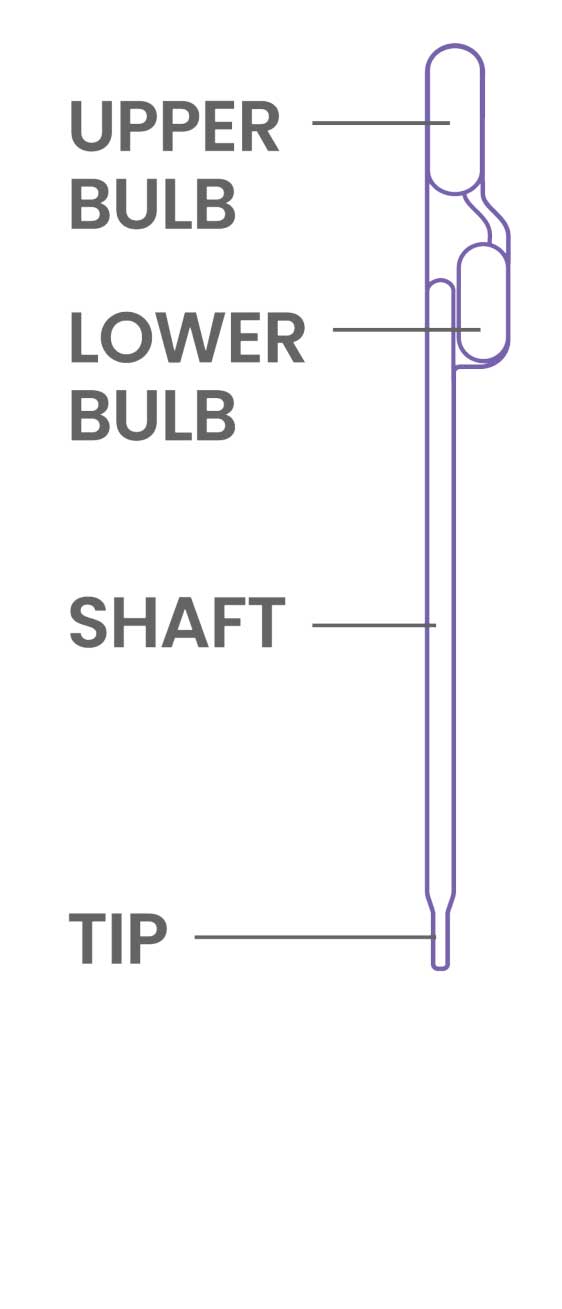
- Treat the pipette similar to an eye dropper, squeeze the upper bulb of the provided pipette prior to submerging the tip to the bottom of the sample tube.
- Do not invert the pipette once the sample is loaded. Doing so may create air bubbles in the shaft.
- Some fluid will remain in the lower bulb of the pipette.
- Once the sample is loaded into the device, keep the upper bulb squeezed as the pipette tip is removed from loading the sample as to not asperate liquid back into the pipette shaft.
- To avoid contamination, do NOT set the pipette down on the counter space. Immediately discard the pipette according to your institution’s guidelines.
Chapter 5 Tips:
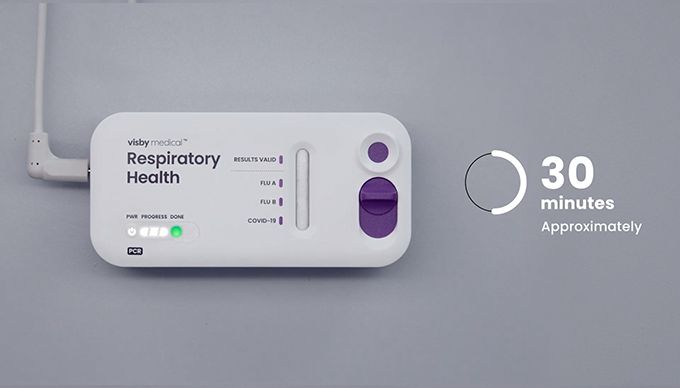
- Once a test is plugged in do not unplug or move the device.
- The results will be ready in approximately 30 minutes. The results are ready when the green “done” light appears (each progress light is approximately in 10 minute increments).
If you are watching this video while running a Visby test, please pause now. After 30 minutes, the test you are running should be complete. When you resume watching, begin with Chapter 6.
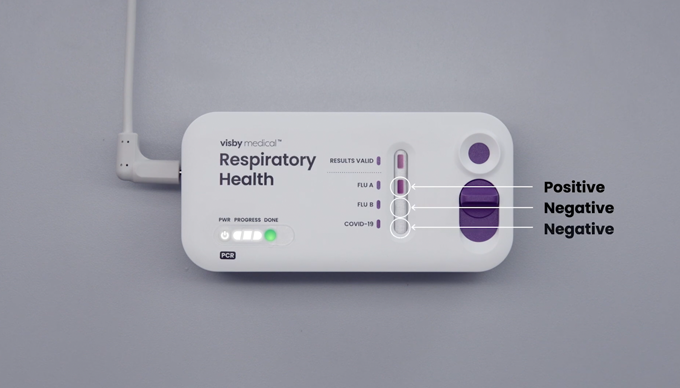
Chapter 6 Tips:
- If a retest is required, use the same patient sample as long as it has been properly stored (≤ 2 hours at room temperature or 48 hours refrigerated) with a new Visby Medical respiratory Health device and new Visby pipette.
- Be sure to invert the specimen 5 times prior to loading the sample into the device.
- Dispose of the device and other materials according to your institution’s guidelines.
For more information, please see the Visby Medical Respiratory Health Test Instructions for Use. Contact us directly by email support@visby.com or call 1-833-468-4729 (1-833-GoVisby) for any additional questions or support.
Please review all Respiratory Health Test chapter videos before taking the quiz.
Quickstart Materials
- Respiratory Health – Quick Reference Guide
- Respiratory Health – Instructions for Use
- Respiratory Health – Patient Collected – Anterior Nasal Collection Instruction- English
- Patient Collected – Anterior Nasal Collection Instructions – Spanish
- Provider Collected – Nasopharyngeal Collection Instructions – English
- Provider Fact Sheet
- Patient Fact Sheet
For all instructional and training materials, please visit our
Playlist
Chapter 1 Tips

- If the pipette has bubbles or an air gap, dispense the sample back into the sample tube and try aspirating again by slowly releasing the upper bulb.
To request additional pipettes for practice, please contact Visby Training.
Chapter 2 Tips:

- Once the sample is loaded into the pipette, do not invert the pipette. Doing so may cause air gaps in the pipette shaft.
- The pipette tip should touch the bottom corner of the well when dispensing a sample into the device.
- Once a sample is loaded into the device, avoid aspirating liquid back into the pipette, by keeping the top bulb of the pipette squeezed as the pipette tip exits the liquid line.
- Immediately dispose the used pipette to avoid contamination.
To request additional pipettes for practice, please contact Visby Training.
Chapter 3 Tips:

- Always visually check there are no air pockets in the shaft of the pipette to ensure a full volume of measurement will be dispensed.
- Once the sample is loaded into the pipette, do not invert the pipette. Doing so may cause air pockets in the pipette shaft.
- Place the pipette tip near the bottom side of the well when dispensing a sample into the device.
- Once a sample is loaded into the device, avoid aspirating liquid back into the pipette, by keeping the top bulb of the pipette squeezed as the pipette tip exits the liquid line.
- Immediately dispose the used pipette to avoid contamination.
To request additional pipettes for practice, please contact Visby Training.